In a somewhat tight-lipped press release and website page, Stryker Orthopaedics notes that it is voluntarily recalling two components of its metal-on-metal hip implant devices. The hip implant recall, according to Stryker, is based on post-market surveillance, which means that there have been a lot of complaints and problems with the devices.
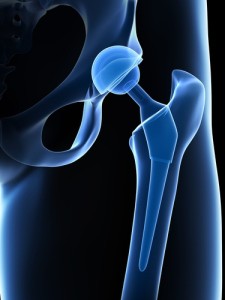 Interestingly, Stryker’s recall is qualified by its statement that “The incidence of complications associated with modular-neck stems is extremely low.” Stryker makes a neat bit of history here by admitting its hip implants are a train wreck while defending the product to the hilt. Kids, don’t try this at home.
Interestingly, Stryker’s recall is qualified by its statement that “The incidence of complications associated with modular-neck stems is extremely low.” Stryker makes a neat bit of history here by admitting its hip implants are a train wreck while defending the product to the hilt. Kids, don’t try this at home.
Anyway, the recalled hip implants include Stryker’s Rejuvenate and ABG II modular-neck stems. These are devices used to help the surgeons correct for anatomical abnormalities, by customizing the implant to the patient. Problems with the devices include corrosion, which can cause inflammation in the body with symptoms of pain and swelling. The original press release for the Rejuvenate hip implant stated that “Laboratory testing demonstrates the compatibility of these materials without concern for fretting and corrosion.”
Regardless of whether patients are experiencing pain or other symptoms, they should contact their orthopedic surgeons to determine whether revision surgery is necessary, or whether the surgeon recommends monitoring of the implant. It’s not a bad idea to contact a lawyer. Medical device lawsuits have strict deadlines and failing to file a lawsuit within a short amount of time means that a lawsuit might not be allowed. For many patients, the clock may tick with this notice of recall.
Stryker set up a website for patients affected by the recall. Not much information there. Shocker.
 Maryland Injury Law Center
Maryland Injury Law Center

